- HHD
- Wuhan Hubei
- In Stock
- In Stock
1. Min 98%
2. Bulk in stock
3. Analysis report offer


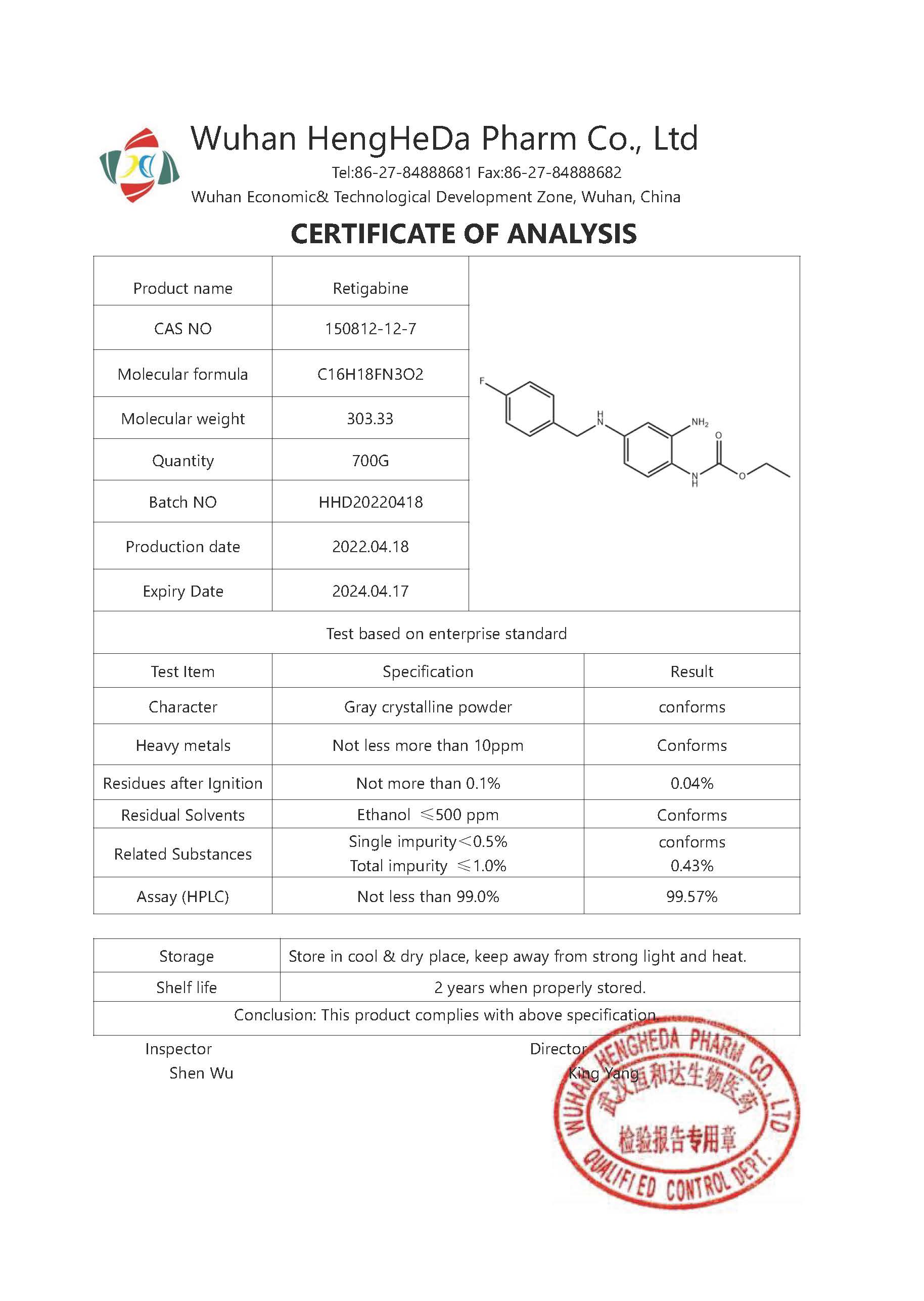
Product Name | Retigabine |
Other Name | Retigabine (Ezogabine) |
CAS No. | 150812-12-7 |
Appearance | White powder |
Purity | 98% |
MF | C16H18FN3O2 |
MW | 303.33 |
Solubility | DMSO: >15mg/mL |
Storage | Room temperature |
Shelf Life | 2 years |
Grade | Food Grade |

Retigabine (INN) or ezogabine (USAN) is an anticonvulsant used as an adjunctive treatment for partial epilepsies in treatment-experienced adult patients.[2] The drug was developed by Valeant Pharmaceuticals and GlaxoSmithKline. It was approved by the European Medicines Agency under the trade name Trobalt on March 28, 2011, and by the United States Food and Drug Administration (FDA), under the trade name Potiga, on June 10, 2011. Production has been discontinued in June 2017.[3][4]
Retigabine works primarily as a potassium channel opener—that is, by activating a certain family of voltage-gated potassium channels in the brain.[5][6][7] This mechanism of action is unique among antiepileptic drugs, and may hold promise for the treatment of other neurologic conditions, including tinnitus, migraine and neuropathic pain. The manufacturer has withdrawn retigabine from clinical use in 2017.
Every batch of our product was tested by authorized independent third party, Analysis testing center, Shanghai branch, Chinese Academy of Science. We send goods to customers with test report and COA. Our products were also tested by American Analytical Chemistry Laboratories and Chromadex too....more